|
||||||||||||
|
||||||||||||
| Research and Development related to Genes for the Creation of New Blood Vessels in its Final Phases | ||||||||||||
| Another area where early application of gene therapy medication is expected is for the treatment of arteriosclerosis obliterans through the neogenesis of blood vessels. Often seen as one of the complications of diabetes, this disease primarily consists of the narrowing and eventual blockage of the arteries supplying blood to the feet (i.e. peripheral vessels). There are many genes related to the regeneration of blood vessels, such as the vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and hepatocyte growth factor (HGF). However, VEGF is the gene for which R&D is being conducted most actively around the world. In the case of Japan, clinical development being conducted in Korea by Takara Bio will soon be entering its final phase. Meanwhile, HGF gene therapy medication that is being developed by AnGes MG, Inc. (a Japanese listed company) is currently also undergoing clinical trials. HGF was discovered in Japan, and AnGes MG holds many of the basic patents. If HGF is approved globally, it will become a major new drug that will result in sales of over 100 billion yen per year. |
||||||||||||
| Great Expectations Also Held for Regenerative Medicine for the Recovery of the Bodily Functions of Elderly People | ||||||||||||
| Regenerative medicine is a technology that utilizes embryonic stem (ES) cells to regenerate organs and tissues, lost through diseases, accidents, etc. or missing through congenital anomalies (birth defects). There are expectations in the application of regenerative medicine not only for the treatment of illnesses but also for the recovery of the bodily functions of elderly people. Kyowa Hakko Kogyo Co., Ltd. is the leader in this field. Kyowa Hakko established a technique for isolating and cultivating mice mesodermal stem cells. Related patents have already been filed and published. There is a strong possibility that it will lead to the discovery of new drug for human use. The practical application of cultured skin is also imminent. Cultured skin has already been commercialized in the U.S. for the treatment of the intractable ulcers of diabetic patients. R&D is being conducted in Japan towards the grafting of skin that has been cultured from the patient's own skin. In the lead in Japan are Japan Tissue Engineering, Ltd. (J-TEC; unlisted company) and BCS, Inc., a venture firm. |
||||||||||||
| The Odds-on Favorite as the Next Generation Biopharmaceutical-based Medication Is Antibody Drugs | ||||||||||||
| Outside of skin culturing, Olympus Corporation is aiming for the practical application of bone engineering technology, while J-TEC is involved in cartilage engineering, etc. Hitachi Medical Corporation is aiming for the practical application of tooth germ regeneration while Terumo Corporation's target is myocardial cell regeneration for coronary heart disease patients. The money is on antibody drugs, however, as being the next-generation biotechnology-based medication. | ||||||||||||
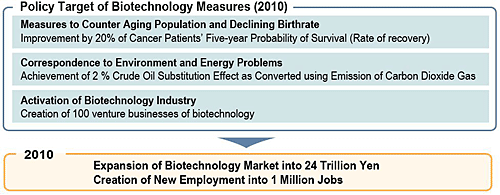 |
||||||||||||
| Biotechnology-based medical research, which utilize genetic engineering to make medication of bioactive proteins, has resulted in the birth of new landmark drugs, beginning with biosynthetic human insulin in the 1980s for the treatment of diabetes and including human-growth hormones, interferon and erythropoietin. Antibody drugs are regarded as odds-on favorites as next-generation biotechnology-based pharmaceutical drugs. Antibodies are proteins that play a central role in the human immune system. They bind to a particular site on an antigen (foreign substances such as viruses, bacteria and toxins), thereby inactivating the antigen and rendering it harmless. By synthesizing antibodies, it will become possible to apply antibodies as medication to boost the immune system or to make a concentrated attack on specific cells. Expectations are placed on the biosynthesis of antibodies for the development of new drugs to be used for such purposes as the treatment of cancer and infectious diseases as well as for transplantation immunology. Chugai Pharmaceutical Co., Ltd., Kirin Brewery Company, Ltd. and Kyowa Hakko are said to be the three leading companies in Japan in relation to antibodies. In the case of Kyowa Hakko, KW-2871, an anticancer agent for the treatment of malignant melanoma, is in the clinical trial phase in the United States. |
||||||||||||
|
|
||||||||||||